The study of molecular structures provides insight into the many functions and behaviours of substances in the field of organic chemistry. One such intriguing molecule, represented by the formula HCOOCH CH2 H2O, has gained notice because of its unusual functional group pairing and possible uses. This article will break down the molecular structure, look at its characteristics, talk about how it is made, and look at potential uses for HCOOCH CH2 H2O.
What is HCOOCH CH2 H2O?
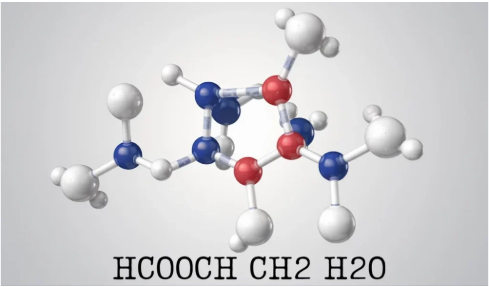
Decomposition of the Molecule
We must dissect HCOOCH CH2 H2O in order to comprehend it better. Important insights are provided by the generic notation:
- HCOO: A carboxylate group (–COO–) joined to a methyl group (–H) is seen in this section.
- CH2: This shows that a methylene bridge, which is frequently present in organic compounds with two carbon atoms connected by a direct single bond, is present in the structure.
- H2O: The presence of water implies that the chemical could be hydrated, which could affect its stability and reactivity.
All things considered, HCOOCH CH2 H2O has an ester-like structure, with the carboxylic acid or ester functionalities potentially playing important roles in solubility and reactivity.
Properties and Structure of Chemicals
The Geometry of Molecular Structure
The chemical behaviour of HCOOCH CH2 H2O is significantly influenced by its shape. The valence shell electron pair repulsion (VSEPR) hypothesis significantly determines the configuration. By identifying the tetrahedral geometry around the carbon atoms and the planarity of the functional groups, we can see the compound’s three-dimensional form. These geometrical factors are important because they influence the polarity, intermolecular interactions, and general reactivity of the molecule.
Physical Characteristics
The structural elements of HCOOCH CH2 H2O can also be used to deduce its physical characteristics. For example:
- Solubility: Because the molecule has a hydrophilic carboxylate group, it should dissolve in water, which makes it a promising option for a number of watery processes.
- Points of Melting and Boiling: Because of hydrogen bonds between water molecules, the presence of both polar and nonpolar components may produce intermediate melting and boiling temperatures.
Methods for the Synthesis of HCOOCH CH2 H2O
HCOOCH can be produced using a variety of synthetic methods, all of which include methodically building the necessary functional groups. Typical synthesis techniques might be:
- Esterification Reactions: When an alcohol and a carboxylic acid react with an acid catalyst present, intermediates that can produce HCOOCH may be produced.
- Esters’ hydrolysis: As an alternative, the target molecule and maybe other byproducts could be produced by hydrolysing an ester in the presence of water.
- Direct Condensation: The production of HCOOCH CH2 H2O can also be achieved by employing techniques such as Michael additions or other condensation processes involving suitable substrates.
Chemical and Pharmaceutical Applications of HCOOCH CH2 H2O
Potential uses in a variety of sectors are made possible by the special structure of HCOOCH CH2 H2O:
- Biological Activity: It is worthwhile to investigate the compound’s reactivity, especially in biological systems. Significant biological activity is frequently exhibited by compounds with carboxylate groups, which may hinder or promote particular biological pathways.
- Material Science: The properties of HCOOCH CH2 H2O may be useful in the production of polymeric materials, adhesives, and coatings where certain hydrophilic or hydrophobic qualities are required.
- Applications in the Environment: Its capacity to dissolve in water raises the possibility that it may be employed in environmental bioremediation to promote biological degradation processes or help contaminants dissolve.
Prospective Research Paths
Even though we now know a lot about HCOOCH CH2 H2O, there are still many chances for research:
- Advanced Characterisation Studies: We can better comprehend HCOOCH CH2 H2O and its interactions by improving molecular characterisation procedures employing spectroscopic techniques including NMR, IR, and UV-Vis.
- Biological Studies: For possible medical uses, it would be helpful to investigate the biological implications of this chemical, including its toxicity and therapeutic potential.
- Synthesis Innovation: Finding novel synthetic routes to produce HCOOCH CH2 H2O more effectively would be very beneficial for both academic and industrial uses.
Conclusion
HCOOCH CH2 H2O is an intriguing chemical that connects a number of important ideas in organic chemistry. There is plenty to investigate, ranging from its synthesis, characteristics, and structural integrity to its possible uses. New discoveries in a range of scientific fields might result from researchers’ ongoing exploration of this compound’s complexities. A comprehensive knowledge of molecules like HCOOCH-CH2-H2O will be essential for unlocking future breakthroughs as we move into an era driven by innovation in chemistry and biology.